Oxygen
2007 Schools Wikipedia Selection. Related subjects: Chemical elements
|
|||||||||||||||||||||||||
| General | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
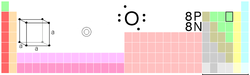
| Name, Symbol, Number | oxygen, O, 8 | ||||||||||||||||||||||||
| Chemical series | Nonmetals, chalcogens | ||||||||||||||||||||||||
| Group, Period, Block | 16, 2, p | ||||||||||||||||||||||||
| Appearance | colorless (gas) very pale blue (liquid)  |
||||||||||||||||||||||||
| Atomic mass | 15.9994 (3) g/mol | ||||||||||||||||||||||||
| Electron configuration | 1s2 2s2 2p4 | ||||||||||||||||||||||||
| Electrons per shell | 2, 6 | ||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||
| Phase | gas | ||||||||||||||||||||||||
| Density | (0 °C, 101.325 kPa) 1.429 g/L |
||||||||||||||||||||||||
| Melting point | 54.36 K (-218.79 ° C, -361.82 ° F) |
||||||||||||||||||||||||
| Boiling point | 90.20 K (-182.95 ° C, -297.31 ° F) |
||||||||||||||||||||||||
| Critical point | 154.59 K, 5.043 MPa | ||||||||||||||||||||||||
| Heat of fusion | (O2) 0.444 kJ·mol−1 | ||||||||||||||||||||||||
| Heat of vaporization | (O2) 6.82 kJ·mol−1 | ||||||||||||||||||||||||
| Heat capacity | (25 °C) (O2) 29.378 J·mol−1·K−1 |
||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||
| Crystal structure | cubic | ||||||||||||||||||||||||
| Oxidation states | −2, −1 (neutral oxide) |
||||||||||||||||||||||||
| Electronegativity | 3.44 (Pauling scale) | ||||||||||||||||||||||||
| Ionization energies ( more) |
1st: 1313.9 kJ·mol−1 | ||||||||||||||||||||||||
| 2nd: 3388.3 kJ·mol−1 | |||||||||||||||||||||||||
| 3rd: 5300.5 kJ·mol−1 | |||||||||||||||||||||||||
| Atomic radius | 60 pm | ||||||||||||||||||||||||
| Atomic radius (calc.) | 48 pm | ||||||||||||||||||||||||
| Covalent radius | 73 pm | ||||||||||||||||||||||||
| Van der Waals radius | 152 pm | ||||||||||||||||||||||||
| Miscellaneous | |||||||||||||||||||||||||
| Magnetic ordering | paramagnetic | ||||||||||||||||||||||||
| Thermal conductivity | (300 K) 26.58 mW·m−1·K−1 | ||||||||||||||||||||||||
| Speed of sound | (gas, 27 °C) 330 m/s | ||||||||||||||||||||||||
| CAS registry number | 7782-44-7 | ||||||||||||||||||||||||
| Selected isotopes | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| References | |||||||||||||||||||||||||
Oxygen ( IPA: /ˈɔksidʒən/) is a chemical element with the chemical symbol O and atomic number 8. On Earth, it is usually covalently or ionically bonded to other elements.
Unbound oxygen (also called molecular dioxygen, O2, a diatomic molecule) first appeared in significant quantities on Earth during the Paleoproterozoic era (between 2.5 billion years ago and 1.6 billion years ago) as a product of the metabolic action of early anaerobes ( archaea and bacteria). The presence of large amounts of free oxygen may have driven most of the organisms then living to extinction. The atmospheric abundance of free oxygen in later geological epochs and up to the present has been largely driven by photosynthetic organisms; roughly three quarters of the free element being produced by algae in the oceans, and one quarter from terrestrial plants.
Characteristics
Oxygen is a major component of air, produced by plants during photosynthesis, and is necessary for aerobic respiration in animals. The word oxygen derives from two roots in Greek, οξυς (oxys) (acid, sharp) and -γενης (-genēs) (born of). In the early 18th century, Antoine Lavoisier coined the name oxygen from the Greek roots mentioned above because he erroneously thought that it was a constituent of all acids. (The definition of acid has since been revised).
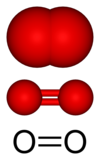
At standard temperature and pressure, oxygen exists as a diatomic molecule with the formula O2, in which the two oxygen atoms are doubly bonded to each other. In its most stable form, oxygen exists as a diradical ( triplet oxygen) with two unpaired electrons in molecular orbitals of the O2 molecule. Though unpaired electrons are commonly associated with high reactivity in chemical compounds, triplet oxygen is relatively (and fortunately) unreactive by comparison with most radicals.
Singlet oxygen, a name given to several higher energy species of molecular oxygen in which all the electron spins are paired, is much more reactive towards common organic molecules. In nature, singlet oxygen is commonly formed from water during photosynthesis, using the energy of sunlight. It is also produced by the immune system as a source of active oxygen. Carotenoids in photosynthetic organisms and possibly also in animals, play a major role in absorbing energy from singlet oxygen and converting it to the unexcited ground state, before it can cause harm to tissues.
Liquid O2 and solid O2 are clear substances with a light sky-blue colour. In normal triplet form they are paramagnetic due to the spin magnetic moments of the unpaired electrons in the molecule, and the negative exchange energy between neighbouring O2 molecules. Liquid oxygen is attracted to a magnet to a sufficient extent that a bridge of liquid oxygen may be supported against its own weight between the poles of a powerful magnet, in laboratory demonstrations. Liquid O2 is usually obtained by the fractional distillation of liquid air.
Oxygen is slightly soluble in water, but naturally occuring disolved amounts support all ocean animal life (see below).
O2 has a bond length of 121 pm and a bond energy of 498 kJ/mol.
Allotropes
Ozone, the triatomic allotrope of oxygen, is a poisonous gas with a sharp odour. It functions in the upper atmosphere of the Earth as a shield against UV radiation, and has recently been found to be produced by the immune system as an antimicrobial (see below). Liquid and solid O3 (ozone) have a deeper blue colour than ordinary oxygen, and they are unstable and explosive.
A recently discovered allotrope of oxygen, tetraoxygen (O4), is a deep red solid that is created by pressurizing O2 to the order of 20 GPa. Its properties are being studied for use in rocket fuels and similar applications, as it is a much more powerful oxidizer than either O2 or O3.
Applications
Oxygen is essential to respiration, so oxygen supplementation has found use in medicine (as oxygen therapy). People who climb mountains or fly in non-pressurized aeroplanes sometimes have supplemental oxygen supplies; the reason is that increasing the proportion of oxygen in the breathing gas at low pressure acts to increase the inspired oxygen partial pressure nearer to that found at sea-level. A notable application of oxygen as a very low-pressure breathing gas, is in modern spacesuits, where use of nearly pure oxygen at a total pressure of about 1/3rd normal, results in normal blood partial pressures of oxygen. This trade-off of breathing gas content and needed pressure is important for space applications, because flexible spacesuits working at Earth sea-level pressures remains a technological challenge beyond today's capabilities.
Oxygen is used in welding (such as the oxyacetylene torch), and in the making of steel and methanol. Liquid oxygen finds use as a classic oxidizer in rocket propulsion.
Oxygen presents two absorption bands centered in the wavelengths 687 and 760 nanometers. Some scientists have proposed to use the measurement of the radiance coming from vegetation canopies in those oxygen bands to characterize plant health status from a satellite platform. This is because in those bands, it is possible to discriminate the vegetation's reflectance from the vegetation's fluorescence, which is much weaker. The measurement presents several technical difficulties due to the low signal to noise ratio and due to the vegetation's architecture, but it has been proposed as a possibility to monitor the carbon cycle from satellites on a global scale.
Oxygen, as a supposed mild euphoric, has a history of recreational use (see oxygen bar), however the reality of this effect is doubtful. Controlled tests of high oxygen mixtures in diving (see nitrox) and other activities, even at higher than normal pressures, show no particular effects on humans other than promotion of an increased tolerance to aerobic exercise.
In the 19th century, oxygen was often mixed with nitrous oxide to promote an analgesic effect; a stable 50% gaseous mixture ( Entonox) is commonly used in medicine today as an analgesic, and 30% oxygen with 70% nitrous oxide is the common basic anaesthetic mixture. These effects, however, are due to the nitrous oxide.
Scientific history
Oxygen was first described by Michał Sędziwój, a Polish alchemist and philosopher in the late 16th century. Sędziwój thought of the gas given off by warm nitre (saltpeter) as "the elixir of life".
Oxygen was more quantitatively discovered by the Swedish pharmacist Carl Wilhelm Scheele some time before 1773, but the discovery was not published until after the independent discovery by Joseph Priestley on August 1, 1774, who called the gas dephlogisticated air (see phlogiston theory). Priestley published discoveries in 1775 and Scheele in 1777; consequently Priestley is usually given the credit. Both Scheele and Priestley produced oxygen by heating mercuric oxide.
Scheele called the gas 'fire air' because it was the only known supporter of combustion. It was later called 'vital air' because it was and is vital for the existence of animal life.
The gas was named by Antoine Laurent Lavoisier, after Priestley's publication in 1775, from Greek roots meaning " acid-former". As noted, the name reflects the then-common incorrect belief that acids contain oxygen.
Occurrence
Oxygen is the most common component of the Earth's crust (49% by mass), the second most common component of the Earth as a whole (28.2% by mass), and the second most common component of the Earth's atmosphere (20.947% by volume), second to nitrogen.
Oxygen occurs as solution in the world's water bodies. At 25° C under 1 atm of air, a litre of water will dissolve about 6.04 cc (8.63 mg, 0.270 mmol) of oxygen, whereas sea water will dissolve about 4.9 cc (7.0 mg, 0.22 mmol). At 0° C the solubilities increase to 10.29 cc (14.7 mg, 0.460 mmol) for water and 8.0 cc (11.4 mg, 0.36 mmol) for sea water. This difference has important implications for ocean life, as polar oceans support a much higher density of life due to their oxygen content.
Compounds
The most familiar of oxygen compounds is water.
Due to its electronegativity, oxygen forms chemical bonds with almost all other elements hence the origin of the original definition of oxidation. The only elements known to escape the possibility of oxidation are a few of the noble gases, and fluorine. Other than water (H2O), well known examples include compounds of carbon and oxygen, such as carbon dioxide (CO2), alcohols (R-OH), carbonyls, (R-CO-H or R-CO-R)), and carboxylic acids (R-COOH). Oxygenated radicals such as chlorates (ClO3−), perchlorates (ClO4−), chromates (CrO42−), dichromates (Cr2O72−), permanganates (MnO4−), and nitrates (NO3−) are strong oxidizing agents in and of themselves. Many metals such as iron bond with oxygen atoms, iron(III) oxide (Fe2O3). Ozone (O3) is formed by electrostatic discharge in the presence of molecular oxygen. A double oxygen molecule (O2)2 is known and is found as a minor component of liquid oxygen. Epoxides are ethers in which the oxygen atom is part of a ring of three atoms.
One unexpected oxygen compound is dioxygen hexafluoroplatinate O2+PtF6−. It was discovered when Neil Bartlett was studying the properties of PtF6. He noticed a change in colour when this compound was exposed to atmospheric air. Bartlett reasoned that xenon should be oxidized by PtF6. This led him to the discovery of xenon hexafluoroplatinate Xe+PtF6−.
Isotopes
Oxygen has seventeen known isotopes with atomic masses ranging from 12.03 u to 28.06 u. Three are stable, 16O, 17O, and 18O, of which 16O is the most abundant (over 99.7%). The radioisotopes all have half-lives of less than three minutes.
An atomic weight of 16 was assigned to oxygen prior to the definition of the unified atomic mass unit based upon 12C. Since physicists referred to 16O only, while chemists meant the naturally abundant mixture of isotopes, this led to slightly different atomic weight scales.
Precautions
Toxicity of O2
Oxygen can be toxic at elevated partial pressures. Since oxygen partial pressure is the fraction of oxygen times the total pressure, elevated partial pressures can occur either from high oxygen fraction in breathing gas, or from high breathing gas pressure, or a combination of both. Oxygen toxicity usually begins to occur at partial pressures more than 0.5 atmospheres, or 2.5 times the normal sea-level oxygen partial pressure of about 0.2 atmospheres or bars. This means that at sea-level pressures, mixtures containing less than 50% oxygen are essentially non-toxic. However in medical applications (such as in ventilation gas mixtures in hospital applications) mixtures containing more than 50% oxygen can be expected to show lung toxicity, causing slow damage to the lungs over periods of days, with the rate of damage rising rapidly from mixtures between 50% and 100% oxygen. On the other hand, breathing 100% oxygen in space applications (such as in some modern spacesuits, or in early spacecraft such as the Apollo spacecraft), causes no damage due to the low total pressures (30% to 33% sea-level) used . In the case of spacesuits, oxygen partial pressure in the breathing gas is typically about 0.30 bar (1.4 times normal), and oxygen partial pressure in the astronaut's blood (due to downward adjustments due to water vapor and CO2 in the alveoli) is close to sea-level normal of 0.14 bar.
In deep scuba diving and surface supplied diving and when using equipment which can provide high partial pressures of oxygen, such as rebreathers, oxygen toxicity to the lungs can occur, just as in medical applications. Due to the higher total pressures in these applications, the fraction of oxygen which produces lung damage may be considerably less than 50%. More importantly, under pressures higher than normal sea-level, a far more serious form of oxygen toxicity in the central nervous system may lead to generalized seizures. This form of oxygen toxicity usually occurs after several hours exposure to oxygen partial pressures over about 1.4 atmospheres (bars) (i.e. 7 times normal), with the time decreasing for higher pressures above this, and with great variation from person to person. At over three bars of oxygen partial pressure (15 times normal), seizures typically occur within minutes.
Toxicity and antibacterial use of other chemical oxygen forms
Certain derivatives of oxygen, such as ozone (O3), singlet oxygen, hydrogen peroxide, hydroxyl radicals and superoxide, are also highly toxic. The body has developed mechanisms to protect against all of these toxic compounds. For instance, the naturally-occurring glutathione can act as an antioxidant, as can bilirubin which is normally a breakdown product of hemoglobin. To protect against the destructive nature of peroxides, nearly every organism on earth has developed some form of the enzyme catalase, which very quickly disproportionates hydrogen peroxide into water and dioxygen. Another nearly universally present enzyme in living organisms (except for a few species of bacteria which use Mn2+ ions directly for the job) is superoxide dismutase. This family of enzymes disproportionates superoxide to oxygen and peroxide, which is then in turn dealt with, by catalase.
Immune systems of higher organisms have long made use of reactive forms of oxygen which they produce. Not only do antibodies catalyze production of peroxide from oxygen, it is now known that immune cells produce peroxide, superoxide, and singlet oxygen in the course of an immune response. Recently, singlet oxygen has been found to be a source of biologically-produced ozone: this reaction proceeds through an unusual compound dihydrogen trioxide, also known as trioxidane, (HOOOH) which is an antibody-catalyzed product of singlet oxygen and water. This compound in turn disproportionates to ozone and peroxide, providing two powerful antibacterials. The body's range of defense against all of these active oxidizing agents is hardly surprising, then, given their "deliberate" employment as antimicrobial agents in the immune response.
Oxygen derivatives are prone to form free radicals, especially in metabolic processes. Because they can cause severe damage to cells and their DNA before they are dealt with, they form part of many theories of carcinogenesis and aging.
Combustion hazard
Highly concentrated sources of oxygen promote rapid combustion and therefore are fire and explosion hazards in the presence of fuels. The fire that killed the Apollo 1 crew on a test launchpad spread so rapidly because the capsule was pressurized with pure oxygen as would be usual in an actual flight, but to maintain positive pressure in the capsule, this was at slightly more than atmospheric pressure instead of the ⅓ normal pressure that would be used in flight. (See partial pressure.)
Similar hazards also apply to compounds of oxygen with a high oxidative potential, such as high concentration peroxides, chlorates, perchlorates, and dichromates; they also can often cause chemical burns.