Prion
2007 Schools Wikipedia Selection. Related subjects: Health and medicine
| ICD- 10 | A81 |
|---|---|
| ICD- 9 | 046 |
A prion ( IPA: [ˈpriːɒn] . listen ) — short for proteinaceous infectious particle (by analogy to virion) — is a type of infectious agent made only of protein. Prions are believed to infect and propagate by refolding abnormally into a structure which is able to convert normal molecules of the protein into the abnormally structured form, and they are generally quite resistant to denaturation by protease, heat, radiation, and formalin treatments, although potency or infectivity can be reduced. The term does not, however, a priori preclude other mechanisms of transmission.
Although genetic research may shed light on prions, and there is a genetic component to many prion diseases, prion diseases are not exclusively genetic diseases. Diseases as varied as fatal familial insomnia and kuru (laughing death) are believed to be associated with prions. Other prion diseases include scrapie (a disease of sheep), chronic wasting disease, (in deer and elk), variant Creutzfeldt-Jakob disease (vCJD), and bovine spongiform encephalopathy (BSE or mad cow disease), all caused by similar proteins in different species. It should be noted that the same gene is responsible for spongiform encephalopathies which are not known to be transmissible, as well as some non-neurological diseases. Some require a mutation for transmission to occur, and there are respective mutations which can prevent transmission for most of the TSEs. A non-disease function of the prion gene is not known but is an area of considerable active research.
All of these diseases affect the structure of the brain or other neural tissue, and all are untreatable and fatal. However, a vaccine has been developed in mice that may provide insight into providing a vaccine in humans to resist prion infections. (see external links below, Science Daily article on vaccine).
Proteins showing prion behaviour are also found in some fungi. Some fungal prions may not be associated with any disease; it is unknown whether these prions represent an evolutionary advantage for their hosts. All known prions are believed to infect and propagate by formation of an amyloid fold, in which the protein polymerizes into a fibre with a core consisting of tightly packed beta sheets. Other mechanisms may exist in yet undiscovered infectious protein particles.
PrP and the prion hypothesis
Radiation biologist Tikvah Alper and physicist J.S. Griffith developed the theory in the 1960s that some TSEs are caused by an infectious agent made solely of protein. This theory was developed to explain the discovery that the mysterious infectious agent causing the diseases scrapie and Creutzfeldt-Jakob Disease, which resisted ultraviolet radiation (which breaks down nucleic acids - present in viruses and all living things), yet responded to agents that disrupt proteins.
A breakthrough occurred in 1982 when researchers led by Stanley B. Prusiner of the University of California, San Francisco purified infectious material and confirmed that the infectious agent consisted mainly of a specific protein. Prusiner coined the word "prion" as a name for the infectious agent, by combining the first two syllables of the words "proteinaceous" and "infectious." While the infectious agent was named a prion, the specific protein that the prion was made of was named PrP, an abbreviation for "protease-resistant protein". Prusiner received the Nobel Prize in Physiology or Medicine in 1997 for this research.
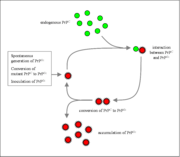
Further research showed that prions are found throughout the body, even in healthy people and animals. However, the prion found in infectious material has a different structure and is resistant to proteases, the enzymes in the body that can normally break down proteins. The normal form of the protein is called PrPC, while the infectious form is called PrPSc— the 'C' refers to 'cellular' PrP, while the 'Sc' refers to ' scrapie,' a prion disease occurring in sheep. Normal prion (Common or cellular) is found on the membranes of cells, though its function has not been fully resolved. Since the original hypothesis was proposed, a gene for the normal protein has been isolated: the PRNP gene.
Some prion diseases (TSEs) can be inherited, and in all inherited cases there is a mutation in the Prnp gene. Many different Prnp mutations have been identified and it is thought that the mutations somehow make PrPC more likely to spontaneously change into the PrPSc (disease) form. TSEs are the only known diseases that can be sporadic, genetic, or infectious; for more information see the article on TSEs.
Although the identity and general properties of prions are now well-understood, the mechanism of prion infection and propagation remains mysterious. It is often assumed that the diseased form directly interacts with the normal form to make it rearrange its structure (enlarge the diagram above for an illustration of this mechanism). One idea, the "Protein X" hypothesis, is that an as-yet unidentified cellular protein (Protein X) enables the conversion of PrPC to PrPSc by bringing a molecule of each of the two together into a complex.
Prior to Alper's insight, all known pathogens (bacteria, viruses, etc.) contained nucleic acids that are necessary for reproduction. The prion hypothesis was highly controversial, because it seemed to contradict the so-called " central dogma of modern biology" that asserts all living organisms use nucleic acids to reproduce. The "protein-only hypothesis" — that a protein structure (which, unlike DNA, has no obvious means of replication) could reproduce itself — was initially met with skepticism. Scientists were skeptical of the idea that a protein structure could reproduce itself without DNA. It does not contradict the central role of DNA. The prion hypothesis proposes a means of spreading the shape of a protein.
Prions in yeast and other fungi
Prion-like proteins that behave in a similar way to PrP are found naturally in some fungi and non-mammalian animals. A group at the Whitehead Institute has argued that some of the fungal prions are not associated with any disease state and may have a useful role, however, researchers at the NIH have also provided strong arguments demonstrating that fungal prions should be considered a diseased state. Research into fungal prions has given strong support to the protein-only hypothesis for mammalian prions, as it has been demonstrated that seeds extracted from cells with the prion state, can convert the normal form of the protein into the infectious form in vitro, and in the process, preserve the information corresponding to different strains of the prion state. It has also shed some light on prion domains, which are regions in a protein that promote the conversion. Fungal prions have helped to suggest mechanisms of conversion that may apply to all prions.
Molecular properties
A great deal of our knowledge of how prions work at a molecular level comes from detailed biochemical analysis of yeast prion proteins. A typical yeast prion protein contains a region ( protein domain) with many repeats of the amino acids glutamine (Q) and asparagine (N); these Q/N-rich domains form the core of the prion's structure. Ordinarily, yeast prion domains are flexible and lack a defined structure. When they convert to the prion state, several molecules of a particular protein come together to form a highly structured amyloid fiber. The end of the fiber acts as a template for the free protein molecules, causing the fiber to grow. Small differences in the amino acid sequence of prion-forming regions lead to distinct structural features on the surface of prion fibers. As a result, only free protein molecules that are identical in amino acid sequence to the prion protein can be recruited into the growing fibre. This "specificity" phenomenon may explain why transmission of prion diseases from one species to another, such as from sheep to cows or from cows to humans is a rare event.
The mammalian prion proteins do not resemble the prion proteins of yeast in their amino acid sequence. Nonetheless, the basic structural features (formation of amyloid fibers and a highly specific barrier to transmission between species) are shared between mammalian and yeast prions. The prion variant responsible for mad cow disease has the remarkable ability to bypass the species barrier to transmission.
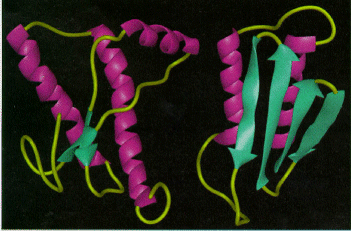
The figure at right shows a model of two conformations of prion; on the left is the known, normal conformation of the structured C-terminal region of PrPC. (to explore/download see the RCSB Protein Databank). The N-terminal region is not shown here for having a flexible structure in aqueous solution. The structured domain shown is mainly made of three spirals called alpha helices (pink), with two short 'flat' regions of beta sheet (β sheet) structure (green). On the right is a proposed model of how the abnormal prion might look. Although the exact 3D structure of PrPSc is not known, there is increased β sheet content (green arrows) in the diseased form of the molecule. These β sheets are thought to lead to amyloid aggregation.
Dissent
Mark Purdy and Doctor David R. Brown have suggested that metal ion interactions with prion protein might be relevant to progression of prion-mediated disease. Purdy cites epidemiological studies of clusters of prion disease in locales with low soil concentrations of copper as evidence.
Brown's work explains how protein tissue can be incinerated and remain "infectious", if that is the proper term for a theory that resembles heavy metal poisoning. Brown, of Oxford, agrees that banning cannibalism in cows was correct.
Therapeutic strategies
Recently Japanese scientists at the Obihiro University of Agriculture and Veterinary Medicine developed one of the first strategies to delaying the onset of disease. They found that sulfated glycosaminoglycans (GAGs) and sulfated glycans inhibit formation of protease resistant protein in cells and prolong the incubation time of scrapie-infected animals. Among the glycopyranosides and their polymers examined, monomeric 4-sulfo-N-acetyl-glucosamine (4SGN), and two glycopolymers, poly-4SGN and poly-6-sulfo-N-acetyl-glucosamine (poly-6SGN), inhibited PrPSc formation with 50% effective doses below 20 microg/ml, and their inhibitory effect became more evident with consecutive treatments. Structural comparisons suggested that a combination of an N-acetyl group at C-2 and an M-sulfate group at either O-4 or O-6 on glucopyranoside might be involved in the inhibition of PrPSc formation. Furthermore, polymeric but not monomeric 6SGN inhibited PrPSc formation, suggesting the importance of a polyvalent configuration in its effect. These results indicate that the synthetic sulfated glycosides are useful not only for the analysis of structure-activity relationship of GAGs but also for the development of therapeutics for prion diseases.
Prion Diseases
The following diseases are now believed to be caused by prions.
- In animals:
- In humans:
-
- several varieties of Creutzfeldt-Jakob Disease (CJD), such as Iatrogenic Creutzfeldt-Jakob disease, Variant Creutzfeldt-Jakob disease, Familial Creutzfeldt-Jakob disease, and Sporadic Creutzfeldt-Jakob disease
- Gerstmann-Sträussler-Scheinker syndrome (GSS)
- Fatal Familial Insomnia (FFI)
- Kuru
- Alpers Syndrome